What is an FDA U.S. Agent?
07/29/2018
Category: Regulatory
Establishment Registration and Product Listings
Before discussion of the role of the FDA U.S. Agent, it is useful to understand Establishment Registrations and Product Listings. These are not the same as FDA approvals, but are nonetheless required. Establishment Registrations and Product Listings exist so that FDA can maintain a catalog of all drug products in commercial distribution in the U.S. Indeed, these requirements apply to most if not all FDA regulated products. The requirements are applicable to, and vary across, animal and veterinary, cosmetics, drugs, food, medical devices, radiation-emitting products, tobacco products and vaccines, blood, and biologics.
Using drugs as the example, “all drug establishments, foreign and domestic, must register their establishments and list all of their drug products in commercial distribution in the United States. The owner or operator of an establishment entering into the manufacture, preparation, propagation, compounding, or processing (which includes, among other things, repackaging and relabeling) of a drug or drugs… In addition, foreign establishments whose products are imported or offered for import into the United States are required to identify a United States agent (only one) during the registration process.”[1]

FDA US Agent
Foreign establishments who import or offer for import into the United States must identify an FDA US Agent during the registration process. The FDA U.S. Agent must be physically located in the United States and will serve as the primary and/or default point of contact between FDA and the firm. The responsibilities of the FDA U.S. Agent are defined in 21 CFR 207.69 as follows:
- Reviewing, disseminating, routing, and responding to all communications from FDA including emergency communications;
- Responding to questions concerning those drugs that are imported or offered for import to the United States;
- Assisting FDA in scheduling inspections; and
- If FDA is unable to contact a foreign registrant directly or expeditiously, FDA may provide the information and/or documents to the United States agent. FDA’s providing information and/or documents to the United States agent is equivalent to providing the same information and/or documents to the foreign registrant.
Official Contact
All drug companies (including U.S. companies) who are subject to registration requirements, whether foreign or domestic must designate an official contact for each establishment. Official Contact responsibilities are also defined in 21 CFR 207.69:
- Ensuring the accuracy of registration and listing information; and
- Reviewing, disseminating, routing, and responding to all communications from FDA including emergency communications.
In order for a drug company to complete an Establishment Registration, 21 CFR 207.25 specifies that the name, mailing address, telephone number, and email address of the Official Contact for the establishment (as provided in 207.69(a)) are required. With respect to foreign establishments the same information must also be provided for the FDA US Agent, as provided in 207.69(b). In finalizing the rules contained within 21 CFR 207 sub-parts A-F, FDA commented that “The information specified in this final rule will help us identify who is manufacturing, repacking, relabeling, or salvaging drugs and where those operations are being performed. In addition, some information (e.g., official contact information) will help us communicate with establishments more effectively and schedule inspections more efficiently.”[2]
eCTD and 356(h)
Foreign firms may designate their US agent of FDA as part of the eCTD submission, by including a letter of appointment in Section 1.3.1.2 (see Figure 1: Excerpt of FDA Comprehensive Table of Contents Headings and Hierarchy). The letter is included as a separate document, and the newly appointed FDA U.S. Agent will also sign Form 356h (See Figure 2: excerpt of FDA form 356h). Note that the form requires both “36. Signature of Applicant’s Responsible Official or Other Authorized Official” and “37. Countersignature of Authorized FDA U.S. Agent”.
Figure 1: Excerpt of FDA Comprehensive Table of Contents Headings and Hierarchy[3]
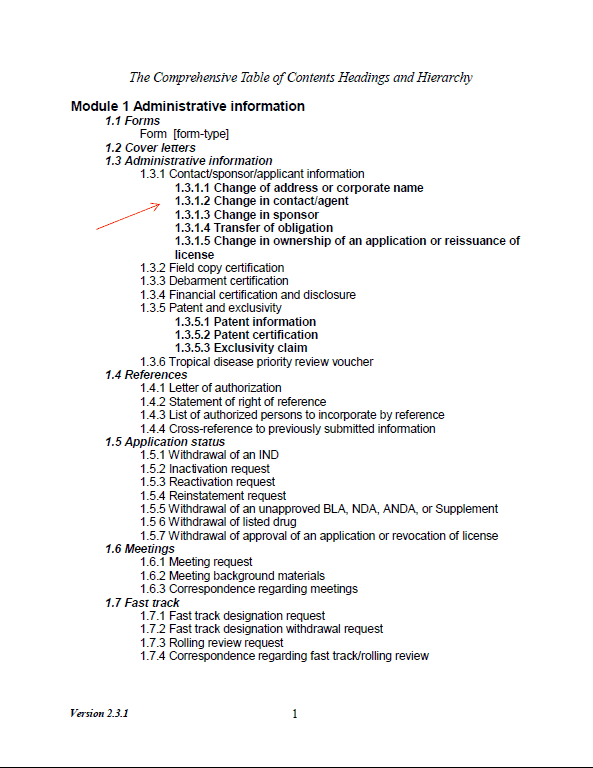
Figure 2: Excerpt of FDA form 356h[4]

When choosing an FDA U.S. Agent, it is important to select someone who brings regulatory insight and understanding to the mix. It is not enough that the FDA U.S. Agent answers the phone, responds to an e-mail or forwards a letter from FDA. Remember, FDA views communications with your FDA U.S. Agent as tantamount to communication with you. Therefore, you need a US Agent of FDA who can answer urgent questions, is current in the regulations and can help you maintain compliance. The synergy of utilizing your regulatory consultant/eCTD submission service as your FDA U.S. Agent should be considered. BRG scientists comprehensively integrate products, therapeutic areas, dosage forms and FDA regulatory pathways.
BRG combines clinical and commercial insights to shepherd the strategic development of drug products, biologics, medical devices, and combination products. Our team possesses extensive experience in the comprehensive integration of products, indications, dosage forms and FDA regulatory pathways. We offer turnkey development assistance to include formulation, medical device design, toxicology, Pharmacokinetics, pharmacodynamics, clinical, statistical, epidemiology, CMC/QSR, labeling and safety surveillance consulting. We source and interact with support organizations worldwide. These include contract laboratories, testing facilities, CROs, CMOs as well as API and component suppliers. Contact BRG to show you the way, or to simply help you along the way.
Notes:
- If I am required to register my drug facility and list my drug product, how do I proceed? as accessed at www.fda.gov on February 1, 2018.
- 81 FR 60169
- The Comprehensive Table of Contents Headings and Hierarchy, Version 2.3.1, page 1 of 42 as accessed at www.fda.gov on February 1, 2018.
- Application to Market a New or Abbreviated New Drug or Biologic for Human Use, Form FDA 356h, page 3 of 3 as accessed at www.fda.gov on February 1, 2018.
BRG is a global pharmaceutical consulting firm with extensive experience in the strategic development of drug products, biologics, medical devices, combination products and in FDA regulatory affairs. As such, BRG is an integral part of client decision-making processes with regard to the designation of FDA U.S. Agents and Official Contacts. In addition, BRG routinely files establishment registrations and product listings, including eCTD and CDER Direct electronic submissions.
tagging: basic > fda compliance training > fda consultant > fda expert witness > fda label > FDA US Agent


