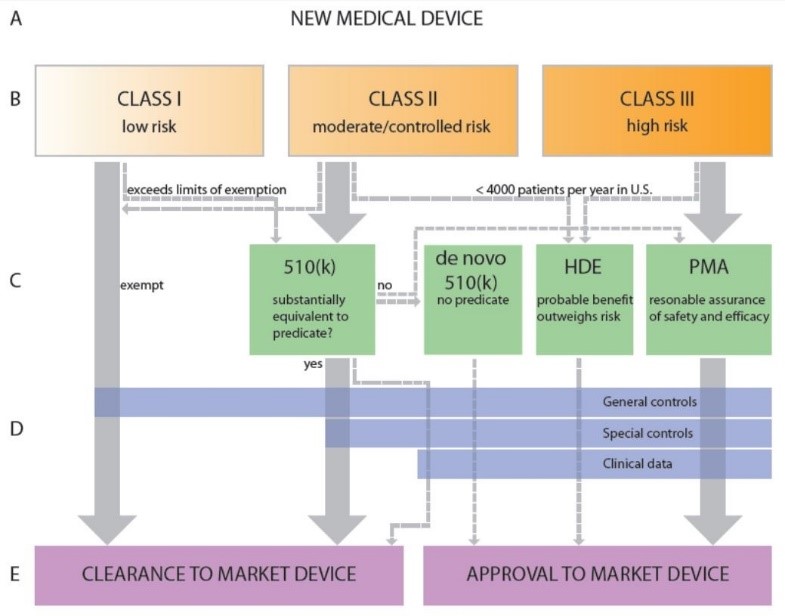
FDA De Novo & Medical Devices
02/02/2022
Category: Medical device consulting
Start Up, Substantial Equivalence (SE), De Novo, and EU-MDR/IVDR Devices have high quality, affordable products that improve the quality of life for...
Read More

